Indian police have arrested the owner of a pharmaceutical company after a cough syrup produced at his factory was linked to the deaths of at least 21 children, authorities said on Thursday.
Most of the victims, all under the age of five, died in the central state of Madhya Pradesh over the past month after taking the syrup, which investigators found to be contaminated with a lethal industrial chemical.
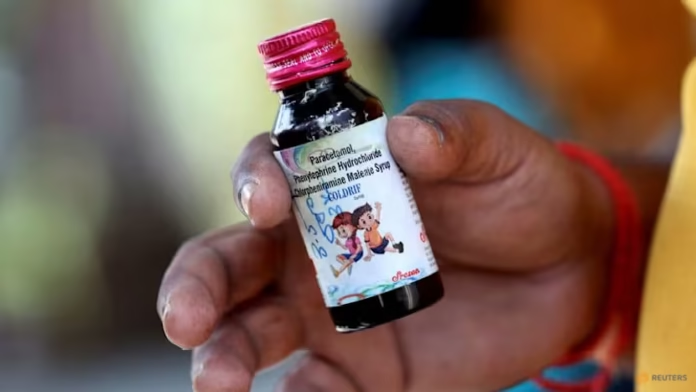
The syrup, sold under the brand name Coldrif, was manufactured by Sresan Pharma at a facility in the southern state of Tamil Nadu.
Police said G. Ranganathan, 75, was detained early Thursday at his home in Chennai in a joint operation by officers from Madhya Pradesh and Tamil Nadu. He faces charges of culpable homicide not amounting to murder and drug adulteration, according to police sources cited by AFP and Indian media.
India’s health ministry said on Saturday that laboratory tests confirmed the syrup contained diethylene glycol (DEG), a toxic substance used in industrial solvents that can cause kidney failure and death even in small quantities. Madhya Pradesh and several other states have since banned the product.
The case has drawn renewed attention to India’s pharmaceutical industry, which has faced growing scrutiny after a series of deadly incidents abroad linked to contaminated cough syrups made in the country.
In 2022, more than 70 children in Gambia died from acute kidney failure after consuming Indian-made cough syrup. Another 68 children in Uzbekistan died between 2022 and 2023 in a similar case involving contaminated medication.
The World Health Organization has reportedly asked Indian authorities to confirm whether the tainted batch of Coldrif syrup was exported.
India is the world’s third-largest drug producer by volume, after the United States and China, according to Reuters.
In January 2023, the U.S. Food and Drug Administration and WHO launched a joint investigation into the global spread of contaminated syrups that have killed more than 300 children across Asia and Africa.
The latest deaths have renewed calls for tighter regulation of India’s drug manufacturing sector, a key supplier to developing nations but one repeatedly rocked by quality-control failures.